Abstract
Introduction: Bosutinib is approved for the treatment of patients with Philadelphia chromosome positive, chronic phase (CP) chronic myeloid leukemia (CML). First-line bosutinib was approved based on the results from the phase 3 BFORE trial, which showed the superior efficacy of bosutinib versus imatinib after ≥12 months of follow-up (Cortes et al., J Clin Oncol 2018). The final efficacy and safety results from BFORE after 5-years of follow-up were recently published (Brümmendorf et al., Leukemia 2022). This analysis investigated the efficacy and safety of bosutinib versus imatinib after 5-years follow-up in a subpopulation of US patients enrolled in the BFORE trial.
Methods: BFORE (NCT02130557) was a global, multicenter, open-label, randomized, phase 3 study of bosutinib versus imatinib. 536 patients with newly diagnosed BCR-ABL1-positive CP-CML were randomized 1:1 to a starting dose of bosutinib or imatinib 400 mg once daily. Study treatment was continued for 5-years or until treatment failure, unacceptable toxicity, death, or withdrawal of consent. Efficacy was assessed by major molecular response (MMR) rate in the intent-to-treat population (all randomized patients). Safety was assessed by the incidence of treatment-emergent adverse events (TEAEs) and permanent treatment discontinuation in the safety population (patients who received ≥1 dose of study treatment). This analysis is based on the final database lock: June 12, 2020, after 5-years (240 weeks).
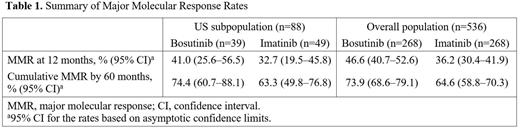
Results: The US subpopulation in BFORE comprised 88 patients (16% of randomized patients); 39 received bosutinib and 49 received imatinib. Baseline disease characteristics were well balanced between treatment arms. The median (range) duration of treatment with bosutinib and imatinib was 54.8 (0.9-58.9) months and 54.8 (1.2-56.4) months, respectively. The median (range) dose intensity with bosutinib and imatinib was 383.5 (162-562) mg/day and 400.0 (189-754) mg/day, respectively. MMR rate at 12 months was 41.0% for bosutinib versus 32.7% for imatinib (Table 1). Cumulative MMR rate by 60 months was 74.4% for bosutinib versus 63.3% for imatinib (Table 1). All patients in both treatment arms experienced at least one TEAE. In the bosutinib arm, the most common TEAEs (≥30% of patients) were diarrhea (84.6%), nausea (61.5%), fatigue (53.8%), thrombocytopenia (41.0%), headache (35.9%), rash (33.3%), increased alanine aminotransferase (ALT; 30.8%), and constipation (30.8%). In the imatinib arm, the most common TEAEs were nausea (63.3%), diarrhea (55.1%), fatigue (36.7%), periorbital edema (34.7%), and muscle spasm (30.6%). 13 (33.3%) patients in the bosutinib arm permanently discontinued treatment due to AEs, versus 7 (14.3%) in the imatinib arm. The most common causes of permanent treatment discontinuation were increased ALT (7.7%) with bosutinib and increased lipase (4.1%) with imatinib. Additional analyses of the US subpopulation are ongoing and will be presented.
Conclusions: The efficacy and safety outcomes in this US subpopulation from BFORE were generally consistent with the overall study population at 5 years (Brümmendorf et al., Leukemia 2022). These results confirm the use of bosutinib as a standard of care in patients with newly diagnosed CP-CML.
Disclosures
Kota:Xcenda: Honoraria; Ariad: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Pfizer Inc: Honoraria, Research Funding. Deininger:Galena Biopharma: Consultancy, Honoraria; Pfizer Inc: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Blueprint Medicines Corporation: Consultancy, Honoraria, Research Funding. Shen:Pfizer Inc: Current Employment, Divested equity in a private or publicly-traded company in the past 24 months. Goldman:Pfizer Inc: Current Employment, Divested equity in a private or publicly-traded company in the past 24 months. Cortes:Gilead: Consultancy; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Sun Pharma: Consultancy, Research Funding; Biopath Holdings Inc: Consultancy, Current equity holder in private company; Abbvie: Consultancy, Research Funding; Forma Therapeutic: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal